New Research Reveals Antifungal Symbiotic Peptide In Legume
Findings pave the way for developing environmentally friendly fungicides
ST. LOUIS, MO, July 20, 2020 – Fungal diseases cause substantial losses of agricultural harvests each year. The fungus Botrytis cinerea causing gray mold disease is a major problem for farmers growing strawberries, grapes, raspberries, tomatoes and lettuce. To mitigate the problem, they often resort to applying chemical fungicides which can lose their effectiveness over time. Danforth Center scientists, Dilip Shah, PhD, research associate member, Siva Velivelli, PhD, postdoctoral associate, Kirk Czymmek, PhD, principal investigator and director, Advanced Bioimaging Laboratory and their collaborators at the Pacific Northwest National Laboratory have identified a sub class of peptides in the nodules of the legume, Medicago truncatula that proved effective in inhibiting growth of the fungus causing gray mold. The results of their research, Antifungal symbiotic peptide NCR044 exhibits unique structure and multifaceted mechanisms of action that confer plant protection, were recently published in the journal, Proceedings of the National Academy of Science.
“We are excited about the possibility of developing this class of peptides as a spray-on fungicide that would provide farmers with an environmentally friendly alternative to chemical fungicides for pre- and post-harvest management of fungal diseases,” said Dilip Shah. “When applied to crops, the peptides will eventually break down to amino acids in the soil and be used by beneficial microbes as an energy source.”
Medicago truncatula is a relative of alfalfa. Shah and his team produced recombinantly large quantities of the highly charged NCR044 peptide that is expressed in the nodules of this legume. They then applied the peptide in low concentrations to tobacco and tomato plants in the lab and challenged the plants with the gray mold fungus. The plants showed significant protection from this fungal disease.
To understand the antimicrobial mechanism within the cell, they collaborated with Czymmek, who is also a mycologist and has studied fungal cell biology for many years. Using time-lapse confocal and super resolution microscopy, the team was able to observe dynamically how the peptide binds to fungal spores and germlings, how it is internalized and where it goes inside the fungal cell. One key finding here was the confirmation that the peptide concentrated in the nucleolus, the organelle where ribosomal assembly takes place.
“It was a pleasure to work with Dilip and his team. As a young scientist, Siva, was able to move diligently across a very diverse set of platforms and techniques, following-up on leads from the scientific data. Ultimately, he was able to apply these corroborating techniques and uncover significant new information to create robust conclusions about the research project,” said Czymmek, “It was really great science.”
The unique team of scientists with expertise in fungal and plant cell biology combined with advanced imaging capabilities allowed them to make critical interpretations and confirm their hypotheses. Their collaborator and co-author on the paper, Garry Buchko, PhD at the Pacific Northwest National Laboratory solved the first three-dimensional structure of a nodule-specific peptide revealing a largely disordered, and highly dynamic, peptide structure containing a short anti-parallel β-sheet, tiny α-helix, and when oxidized, two stabilizing disulfide bonds.
A portion of the project was funded by TechAccel. Shah and Czymmek will continue their research and have applied to the National Science Foundation for a grant to further explore how antifungal nodule-specific peptides kill harmful fungal pathogens in vitro and in planta.
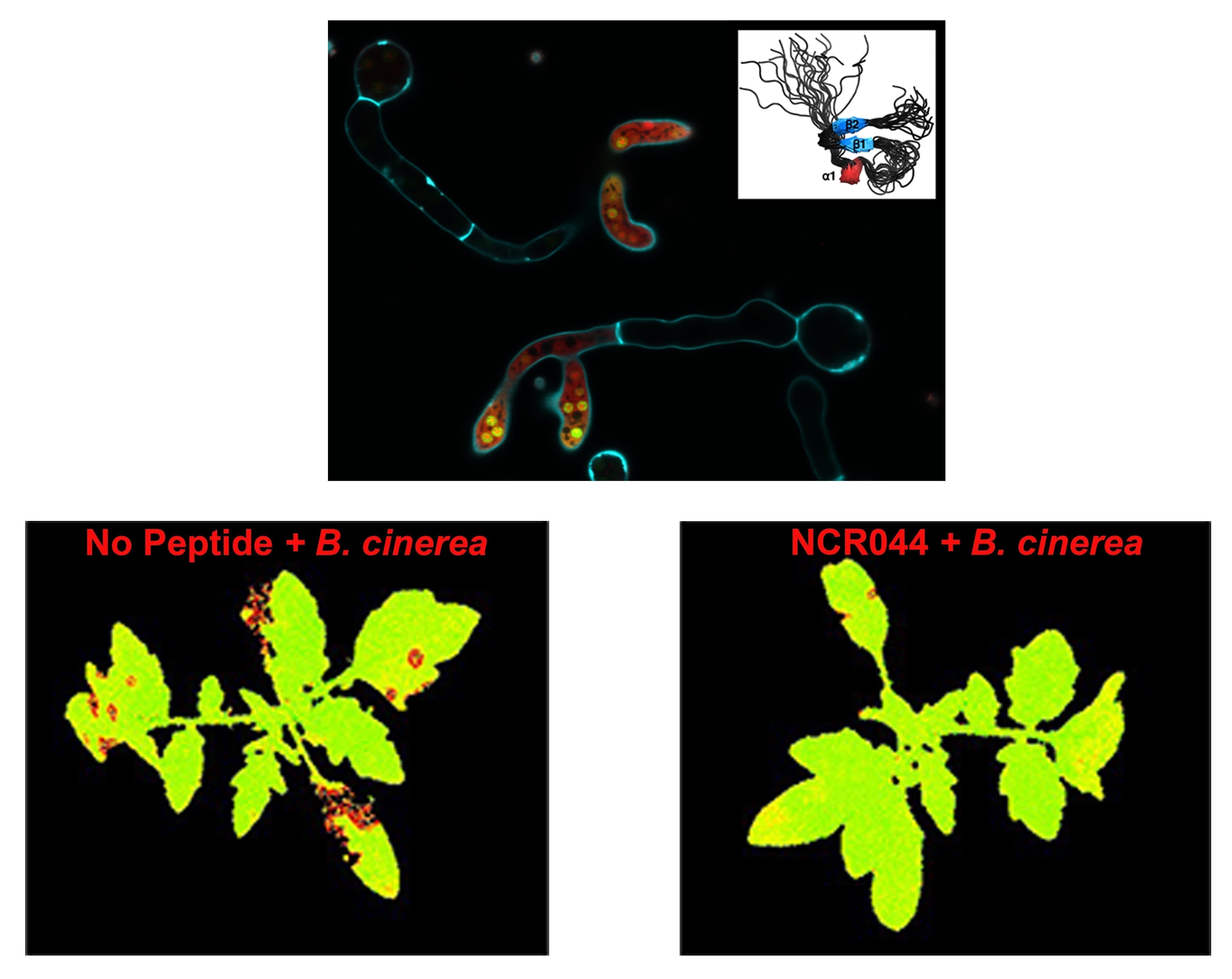
Image courtesy of Velivelli et. al., (PNAS) and Advanced Bioimaging Laboratory, Donald Danforth Plant Science Center. A peptide-based antifungal agent (three-dimensional structure shown in the upper right corner), when fluorescently labeled with red dye and visualized using confocal-microscopy, becomes dispersed in the cytoplasm and accumulates in the nucleolus of a common plant fungal pathogen, Botrytis cinerea. Calcofuor white (cyan) was used to stain the cell wall and septa of fungal cells. Tomato plants sprayed with fungal spores show areas of reduced photosynthesis (falsely colored red) due to damage from the pathogen (bottom left). Plants sprayed with the antifungal peptide NCR044 are protected from fungal damage (bottom right).
Images are available upon request.
About The Donald Danforth Plant Science Center
Founded in 1998, the Donald Danforth Plant Science Center is a not-for-profit research institute with a mission to improve the human condition through plant science. Research, education and outreach aim to have impact at the nexus of food security and the environment, and position the St. Louis region as a world center for plant science. The Center’s work is funded through competitive grants from many sources, including the National Institutes of Health, U.S. Department of Energy, National Science Foundation, and the Bill & Melinda Gates Foundation. Follow us on Twitter at @DanforthCenter.
